Project:Growing bacteria: Difference between revisions
Mycoplasma (talk | contribs) (Created page with "=== 7th march === - Two plates of v. fischeri put in lab. They are bioluminescent and glow green in the dark. They had been grown for a few days on 'boss' media === 9th march ...") |
m (Montyphy moved page Growing bacteria to Project:Growing bacteria) |
||
| (47 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
== V. fischeri + E. coli == | |||
=== V. fischeri life cycle + plating techniques === | |||
* Apparently they glow brighter in the evening. | |||
* No or faint brightness on day 1. Peak brightness on day 2-4. Faint by day 5. Faint glow can remain until day 8. | |||
* So far no frozen cultures have been revived. | |||
* BOSS media still works after being frozen. | |||
* Media can be boiled. | |||
* Recipe: 2% table salt, 2.5% LB, 1.5% agar. For 100ml of BOSS media this would be 2g table salt, 2.5g LB, 1.5g agar in 100ml dH20. | |||
=== 7th march === | === 7th march === | ||
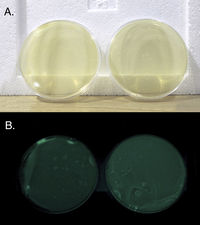
[[File:Glowing_plates.jpg|200px|thumb|left|Photo by Raphael Kim]] | |||
Two plates of v. fischeri brought to lab. They are bioluminescent and glow green in the dark. When we got them they had been growing for a few days on 'BOSS' media (BOSS medium: 1% peptone, 0.3% beef extract, 0.1% glycerol, 3% NaCl, 1.5% agar, pH 7.3) | |||
<br style="clear: both" /> | |||
=== 9th march === | === 9th march === | ||
Some cultures transferred to new plates | Some cultures transferred to new plates. [http://wiki.london.hackspace.org.uk/view/File:Vibrio_Fischeri_Culture_Protocol_9.3.pdf Protocol] | ||
=== 10th march === | |||
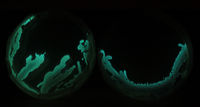
[[File:V.fisch_culturing.JPG|200px|thumb|left|Photo by Raphael Kim]] | |||
More cultures transferred to new plates. [http://wiki.london.hackspace.org.uk/view/File:10.3.13.pdf Protocol] | |||
<br style="clear: both" /> | |||
===13th march=== | ===13th march=== | ||
Original cultures still | Original cultures still glowing strongly, but nothing from the new ones. | ||
===15th march=== | |||
Still nothing from new cultures. Original culture A was still going strong, Culture B is much weaker. | |||
===16th march=== | |||
Culture A still ok, culture B almost disappeared. | |||
Two cultures of ''V. fischeri'', two cultures of ''E. coli'', and one culture of 'biohackers thumb prints' were plated onto an approximation of BOSS media. Media was 4g table salt, 5g LB (containing peptone, salt and yeast), 0.2g glycerol, 3g agar in 200ml water. (3% nacl, 1% peptone, 0.1% glycerol, 0.5% yeast extract, 1.5% agar). Some cultures of ''V. fischeri'' from original plate A were frozen in the same medium minus the agar, and with 3% glycerol instead of 0.1%. Cultures stored at room temp, although ''E. coli'' incubated at 37C for a couple of hours first. | |||
===18th march, 1pm=== | |||
[[File:Bacteria_plates.jpg|200px|thumb|left|Photo by Millie Clive-Smith]] | |||
Checked the plates, the two new V. fischeri cultures are glowing very strongly on the first areas streaked. Can't see much from anything else. | |||
<br style="clear: both" /> | |||
===22th march=== | |||
3 new plates of V. fischeri made up | |||
===26th march=== | |||
* Fisch plate A from 16/3 glowing faintly in parts, plate B glowing very faintly all over | |||
* Fisch plates 1,2 and 3 from 22/3 glowing strongly | |||
* E. coli plates from 16/3 seem to have good growth | |||
===27th march (Lui & David)=== | |||
* Fisch plates 1 and 3 from 22/3 glowing, 2 not glowing | |||
* 3 new plates of V. fischeri made up from 22/3 plates. 1 from each. 3 plates of BOSS media frozen. | |||
===2nd April 4pm (Lui)=== | |||
* Three most recent Vibrio Fischeri plates are glowing faintly (A27Mar, B27Mar, C27Mar). | |||
* Only one blue rod was left, so I made only one new sample (A2Apr) from the brightest existing sample (A27Mar), using a plate of frozen BOSS media. | |||
* There are still two frozen BOSS media plates in the freezer. | |||
===4th April 6pm (Will)=== | |||
* Three 27th Vibrio Fischeri plates still glowing (A27Mar, B27Mar, C27Mar). | |||
* A2Apr no longer glowing | |||
* Frozen BOSS defrosted and put into two new plates. New plate made up from A27Mar. | |||
===11th April 7:30pm=== | |||
* No Vibrio Fischeri plates still glowing except for A4apr faintly | |||
* Two new plates made up from boss media in freezer. (Media was melted and resolidified). One streaked from frozen culture in freezer, the other streaked from A4Apr | |||
===14th April=== | |||
* No Vibrio Fischeri plates still glowing except for A4apr very very faintly. (So neither plate from 11Apr showed anything) | |||
* Two new plates made up from fresh BOSS media. Both (A14Apr and B14Apr) streaked from A4Apr | |||
===16th April=== | |||
* A14Apr and B14Apr glowing well | |||
===17th April 19:00=== | |||
* A14Apr and B14Apr glowing well | |||
===19th April 18:00 === | |||
* A14Apr and B14Apr glowing faintly. Two new plates (A20apr and B20apr) made up with fresh BOSS from A14Apr | |||
===20th April 18:00 === | |||
* A14Apr and B14Apr not glowing, A20apr and b20apr not glowing. Made A21apr from A14apr and frozen 20apr BOSS, B21apr from A20apr and fresh BOSS. | |||
===26th April 18:00 === | |||
* A20apr and B20apr glowing, A21apr and B21apr glowing. | |||
===27th April 18:00 === | |||
* A20apr and B20apr glowing, A21apr and B21apr glowing. | |||
===30th April 18:00 === | |||
* A20apr and B20apr not glowing, A21apr and B21apr glowing faintly. Made A30Apr and B30Apr with respective plates from 21Apr, using frozen BOSS from 21Apr. | |||
===7th May afternoon === | |||
* A30Apr and B30Apr glowing very faintly | |||
===8th May evening === | |||
* A30Apr and B30Apr not glowing. Made A8May and B8May from them. | |||
===11th May afternoon === | |||
* A8May and B8May glowing. Made 4 liquid cultures from A8May, snap froze them with LN2 and stored at -20C | |||
== Equipment == | |||
We need to fix our incubator with shaker, or make a new one. | |||
====Requirements==== | |||
* Easily cleaned + sterilised | |||
* Either a shaker powerful enough to agitate full beakers, or a magnetic stirrer (which we have already started building). Magnetic stirrer may lyse cells. An idea for a shaker could be a record player. | |||
* Can be made dark inside | |||
* Adjustable temp | |||
* Low power if possible. | |||
* Temperature mesaurement (simple thermometer would do) | |||
* Does it need to be sealable? | |||
[[Category:Biohacking]] | |||
[[Category:Projects]] | |||
Latest revision as of 15:11, 3 June 2013
V. fischeri + E. coli
V. fischeri life cycle + plating techniques
- Apparently they glow brighter in the evening.
- No or faint brightness on day 1. Peak brightness on day 2-4. Faint by day 5. Faint glow can remain until day 8.
- So far no frozen cultures have been revived.
- BOSS media still works after being frozen.
- Media can be boiled.
- Recipe: 2% table salt, 2.5% LB, 1.5% agar. For 100ml of BOSS media this would be 2g table salt, 2.5g LB, 1.5g agar in 100ml dH20.
7th march
Two plates of v. fischeri brought to lab. They are bioluminescent and glow green in the dark. When we got them they had been growing for a few days on 'BOSS' media (BOSS medium: 1% peptone, 0.3% beef extract, 0.1% glycerol, 3% NaCl, 1.5% agar, pH 7.3)
9th march
Some cultures transferred to new plates. Protocol
10th march
More cultures transferred to new plates. Protocol
13th march
Original cultures still glowing strongly, but nothing from the new ones.
15th march
Still nothing from new cultures. Original culture A was still going strong, Culture B is much weaker.
16th march
Culture A still ok, culture B almost disappeared. Two cultures of V. fischeri, two cultures of E. coli, and one culture of 'biohackers thumb prints' were plated onto an approximation of BOSS media. Media was 4g table salt, 5g LB (containing peptone, salt and yeast), 0.2g glycerol, 3g agar in 200ml water. (3% nacl, 1% peptone, 0.1% glycerol, 0.5% yeast extract, 1.5% agar). Some cultures of V. fischeri from original plate A were frozen in the same medium minus the agar, and with 3% glycerol instead of 0.1%. Cultures stored at room temp, although E. coli incubated at 37C for a couple of hours first.
18th march, 1pm
Checked the plates, the two new V. fischeri cultures are glowing very strongly on the first areas streaked. Can't see much from anything else.
22th march
3 new plates of V. fischeri made up
26th march
- Fisch plate A from 16/3 glowing faintly in parts, plate B glowing very faintly all over
- Fisch plates 1,2 and 3 from 22/3 glowing strongly
- E. coli plates from 16/3 seem to have good growth
27th march (Lui & David)
- Fisch plates 1 and 3 from 22/3 glowing, 2 not glowing
- 3 new plates of V. fischeri made up from 22/3 plates. 1 from each. 3 plates of BOSS media frozen.
2nd April 4pm (Lui)
- Three most recent Vibrio Fischeri plates are glowing faintly (A27Mar, B27Mar, C27Mar).
- Only one blue rod was left, so I made only one new sample (A2Apr) from the brightest existing sample (A27Mar), using a plate of frozen BOSS media.
- There are still two frozen BOSS media plates in the freezer.
4th April 6pm (Will)
- Three 27th Vibrio Fischeri plates still glowing (A27Mar, B27Mar, C27Mar).
- A2Apr no longer glowing
- Frozen BOSS defrosted and put into two new plates. New plate made up from A27Mar.
11th April 7:30pm
- No Vibrio Fischeri plates still glowing except for A4apr faintly
- Two new plates made up from boss media in freezer. (Media was melted and resolidified). One streaked from frozen culture in freezer, the other streaked from A4Apr
14th April
- No Vibrio Fischeri plates still glowing except for A4apr very very faintly. (So neither plate from 11Apr showed anything)
- Two new plates made up from fresh BOSS media. Both (A14Apr and B14Apr) streaked from A4Apr
16th April
- A14Apr and B14Apr glowing well
17th April 19:00
- A14Apr and B14Apr glowing well
19th April 18:00
- A14Apr and B14Apr glowing faintly. Two new plates (A20apr and B20apr) made up with fresh BOSS from A14Apr
20th April 18:00
- A14Apr and B14Apr not glowing, A20apr and b20apr not glowing. Made A21apr from A14apr and frozen 20apr BOSS, B21apr from A20apr and fresh BOSS.
26th April 18:00
- A20apr and B20apr glowing, A21apr and B21apr glowing.
27th April 18:00
- A20apr and B20apr glowing, A21apr and B21apr glowing.
30th April 18:00
- A20apr and B20apr not glowing, A21apr and B21apr glowing faintly. Made A30Apr and B30Apr with respective plates from 21Apr, using frozen BOSS from 21Apr.
7th May afternoon
- A30Apr and B30Apr glowing very faintly
8th May evening
- A30Apr and B30Apr not glowing. Made A8May and B8May from them.
11th May afternoon
- A8May and B8May glowing. Made 4 liquid cultures from A8May, snap froze them with LN2 and stored at -20C
Equipment
We need to fix our incubator with shaker, or make a new one.
Requirements
- Easily cleaned + sterilised
- Either a shaker powerful enough to agitate full beakers, or a magnetic stirrer (which we have already started building). Magnetic stirrer may lyse cells. An idea for a shaker could be a record player.
- Can be made dark inside
- Adjustable temp
- Low power if possible.
- Temperature mesaurement (simple thermometer would do)
- Does it need to be sealable?