E. coli transformation: Difference between revisions
From London Hackspace Wiki
Mycoplasma (talk | contribs) No edit summary |
Mycoplasma (talk | contribs) (→21 Apr) |
||
| Line 22: | Line 22: | ||
* Incubated at 98C for 15 minutes, then hold at 10C (boiling program in PCR machine) - to lyse the cells | * Incubated at 98C for 15 minutes, then hold at 10C (boiling program in PCR machine) - to lyse the cells | ||
* Put on 2 PCRs on Col program (53C annealing) - one with M13 primers (for PUC19), one with S16 primers (for any bacteria), then hold for 4C for 20 hours. (Sam said 56 might be better for M13 but is too hot for s16) | * Put on 2 PCRs on Col program (53C annealing) - one with M13 primers (for PUC19), one with S16 primers (for any bacteria), then hold for 4C for 20 hours. (Sam said 56 might be better for M13 but is too hot for s16) | ||
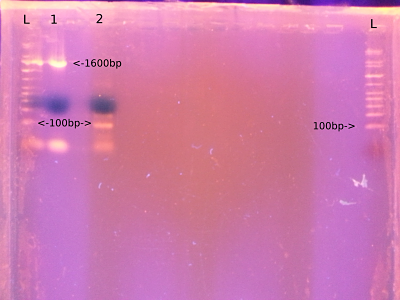
* From | * From left - ladder, M13, S16, ladder | ||
=== 28 Apr === | === 28 Apr === | ||
Revision as of 09:46, 2 May 2015
E coli transformed with PUC19 using ampicillin selection and electroporation
See here for protocol, successfully used by Tom H.
28 mar 15
- 2ml ecoli culture (bottle on shelf) incubated in 200ml LB (bottle in fridge)
- -ve control is untransformed ecoli from same culture put on LBA
- +ve control is transformed ecoli from same culture put on LB plates
- Transform ecoli with PUC19 using electroporation
- Plate onto LBA.
- No e coli survived on plates, whether with Amp or not. Could be various reasons.
12 Apr 15
- Following main gdocs protocol above. Initial 2x100ml cultures started off at 14:40.
- Ended up with some growth on control amp plate as well as transformed amp plate. Will do colony PCR and check if it worked with M13
21 Apr
- Took 1 colony from PUC19 dh5a plate and mixed with 250ul RO water
- Incubated at 98C for 15 minutes, then hold at 10C (boiling program in PCR machine) - to lyse the cells
- Put on 2 PCRs on Col program (53C annealing) - one with M13 primers (for PUC19), one with S16 primers (for any bacteria), then hold for 4C for 20 hours. (Sam said 56 might be better for M13 but is too hot for s16)
- From left - ladder, M13, S16, ladder
28 Apr
- Ran on a gel. Pic to come.
- Expect M13 product of about 120bp
GFP transformation
Growing chemically competent E. coli
Here is the protocol we used with UCL to grow competent cells during the making of the public biobrick. So we need to get E. coli (one possibility. Could also try NCBE. They sell e. coli as a transformer kit replacement part. Finally these are suggested for schools by NCBE: Sciento, Blades Biological and Philip Harris Education), LB - £66, 0.1M CaCl2 - £32.50, M9 salts - £57.60, MgSO4 - £20, bacteriological agar solution - £42.90, thiamine - ~£20, D glucose - ~£20, and plates. All links are only the first ones I found, maybe cheaper options available.