E. coli transformation: Difference between revisions
From London Hackspace Wiki
Mycoplasma (talk | contribs) (→28 Apr) |
Mycoplasma (talk | contribs) (→28 Apr) |
||
| Line 39: | Line 39: | ||
* Replated both transformed and untransformed dh5a on amp plates | * Replated both transformed and untransformed dh5a on amp plates | ||
[ | [http://authorherstorianparent.blogspot.co.uk/2011/11/henry-viis-half-hearted-attempts-to-woo.html Perfectsize 1KB XL] | ||
=== 29 Apr === | === 29 Apr === | ||
Latest revision as of 10:32, 2 May 2015
E coli transformed with PUC19 using ampicillin selection and electroporation
See here for protocol, successfully used by Tom H.
28 mar 15
- 2ml ecoli culture (bottle on shelf) incubated in 200ml LB (bottle in fridge)
- -ve control is untransformed ecoli from same culture put on LBA
- +ve control is transformed ecoli from same culture put on LB plates
- Transform ecoli with PUC19 using electroporation
- Plate onto LBA.
- No e coli survived on plates, whether with Amp or not. Could be various reasons.
12 Apr 15
- Following main gdocs protocol above. Initial 2x100ml cultures started off at 14:40.
- Ended up with some growth on control amp plate as well as transformed amp plate. Will do colony PCR and check if it worked with M13
21 Apr
- Took 1 colony from PUC19 dh5a plate and mixed with 250ul RO water
- Incubated at 98C for 15 minutes, then hold at 10C (boiling program in PCR machine) - to lyse the cells
- Put on 2 PCRs on Col program (53C annealing) - one with M13 primers (for PUC19), one with S16 primers (for any bacteria), then hold for 4C for 20 hours. (Sam said 56 might be better for M13 but is too hot for s16)
- M13 rev (-29) 5'-CAGGAAACAGCTATGACC-3'
- M13 uni (-21) 5'-TGTAAAACGACGGCCAGT-3'
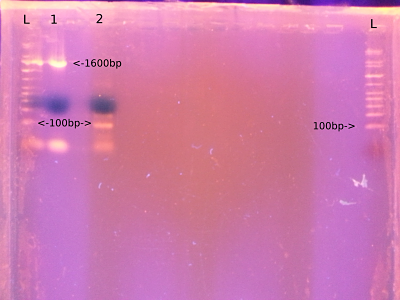
- From left - ladder, M13, S16, ladder
28 Apr
- Ran on a 1% agarose gel. 115V for 45 mins
- Ladder was Perfectsize 1KB XL
- Visualised on transilluminator
- Expect M13 product of 104bp
- Replated both transformed and untransformed dh5a on amp plates
29 Apr
- Growth on both plates. Either transformed contamination on control plate (most likely), or bad AMP. Need to do PCR / miniprep to confirm.
GFP transformation
Growing chemically competent E. coli
Here is the protocol we used with UCL to grow competent cells during the making of the public biobrick. So we need to get E. coli (one possibility. Could also try NCBE. They sell e. coli as a transformer kit replacement part. Finally these are suggested for schools by NCBE: Sciento, Blades Biological and Philip Harris Education), LB - £66, 0.1M CaCl2 - £32.50, M9 salts - £57.60, MgSO4 - £20, bacteriological agar solution - £42.90, thiamine - ~£20, D glucose - ~£20, and plates. All links are only the first ones I found, maybe cheaper options available.