CL1 BSO SOP GLP
CL1, BSO, SOP, GLP .. regulations and notification process
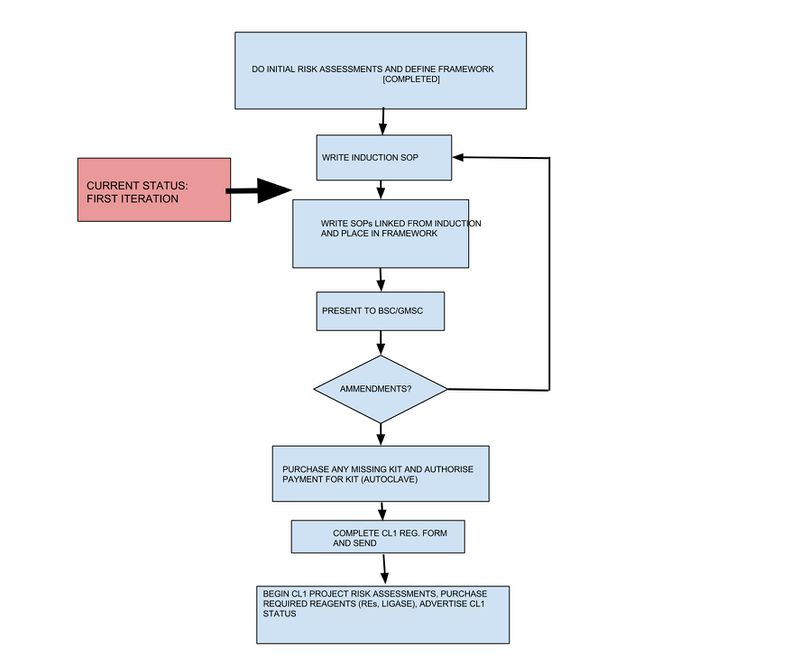
The purpose here is to take all the previous info regarding CL1, biolab health & safety, GLP guides and biolab protocols etc and boil it down into documentation suitable to support our CL1 notification.
It may seem wordy etc, but that's the nature of these regs in general and is part of the process. Not only will we be confident in our application but we also make the biolab at the hackspace safe and accessible for those wanting to work on bio projects.
Things done:
1) Lab risk assessment (see below)
2) Define SOP framework: BioLabSOPs
2a) Create Induction procedure for new biolab people (see LBL02001)
... got equipment: gel box and autoclave!
2b) Here is an SOP Template, containing instructions for each section so that future users can write SOPs for this framework...
Things to do:
3) Risk assessments for new biolab projects and the formation of biosafety/GM committee, enactment of BSO (Will be in BioLab SOP sections 3,4,5 and 6
4) SOPs TODO (once we have SOPs in the framework for these we can get the SC involved)
project risk assessment... LBL06001 Project Risk Assessment Procedure for Lab Users Proposing New Projects, (in 3, above, a specific example of this for one or more projects in current operation would be good/essential)
prep of gels.... LBL07003 Gel preparation and electrophoresis
electrophoresis... LBL07003 Gel preparation and electrophoresis
operating spectrophotometer... LBL04001 Operating the Spectrophotometer
operating PCR machine... LBL07004 Setting up and running PCRs e.g Colony PCR
setting up PCRs... LBL07004 Setting up and running PCRs e.g Colony PCR
EtBr handling and storage... LBL07005 Handling and Storage of Ethidium Bromide (EtBr)
UV vis illuminator usage... LBL07003 Gel preparation and electrophoresis
HEPA flow hood usage... LBL04005 Operating the Laminar Flow Unit for asceptic sample handling
Autoclave usage... LBL04002 Using the Autoclave for Media Preparation and Waste Deactivation
General maintenance/cleaning/washing up... LBL08001 Maintenance and Cleaning
Chemical storage... LBL0A001 BioLab Chemical Storage, LBL0A002 BioLab Chemicals MSDS Catalogue
Incubator usage... LBL04004 Using The Incubator
Operating centrifuges... LBL04003 The BioLab Centrifuges and their usage
Sample and reagent storage, fridge and freezer
roles of BSO... LBL03001 The Roles of the BSO and Safety Committee
roles of BS and GM SC... LBL03001 The Roles of the BSO and Safety Committee
update induction to include new autoclave info
This may or may not explain things better:
London Hackspace BioLab Risk Assessment
Laboratory Access Control
A suitable system is in place to control the access to the laboratory area within the building in order to ensure that only trained and responsible individuals can make use of the laboratory facilities of which this risk assessment is primarily concerned. Training consists firstly of a process of induction laid out in the LBL Standard Operating Procedures (SOPs) and further requires that users of the lab have considered the health and safety implications of the work to be carried out, given the organisms, reagents, procedures and skill level required. The Biological Safety Officer (BSO) and Biological Safety and Genetic Modification Safety (where relevant) Committees should be be informed of the nature of the work any users of the laboratory wish to undertake. Full details of the requirements of of principal investigators can also be found in the SOPs.
PPE, Biosafety and Contamination Risks
The biological organisms for which work will be carried out within the laboratory include
i) non-pathogenic strains of E. coli (such as DH5 alpha),
ii) cellulose producing gram-negative bacteria such as Gluconacetobacter,
iii) non-toxic Dinoflagelates,
iv) brewer’s yeast (Saccharomices cereviscae) and,
v) algal microorganisms from non-toxic environmental samples.
None of organisms for which the laboratory is being used to study require containment level (CL) precautions above that of CL1.
Those which fall into the classification of CL1 requirements include only those strains genetically modified by transformation. In particular, the transformation of E. coli by heat shock method and of Gluconacetobacter by electroporation. Both of these procedures are explained in the SOPs.
Personal Protective Equipment (PPE) suitable for working with these kinds of organisms is provided and consists of lab coats, goggles and gloves. If suitable PPE is not available to hand the BSO should be informed immediately before commencing any practical work.
To ensure that any contamination of genetically modified biomaterial and microorganisms does not occur, laboratory users should be aware of the correct handling and disposal procedures required. They should be made aware of typical requirements during laboratory safety induction and this knowledge should be reinforced during individual project risk assessments. Generally, all microorganisms should be handled while using gloves, eye protection and protective clothing to prevent material coming into contact with the user’s skin or eyes. This is both to protect the user and to eliminate the risk of transporting biomaterial out of the laboratory after work is completed. Gloves can be disposed of in the bins provided. Containment level 1 biomaterial should be deactivated before disposal by autoclaving and immersion in bleach in the ‘kill bin’ underneath the sink. The deactivated waste in the kill bin can then be diluted further and disposed of down the drain
Microwave
The microwave is a familiar device to probably all laboratory users and the standard precautions apply when using it. In particular, the handling of hot liquids and glassware may present the risk of burns and as such thermally resistant gloves are provided. Of note is also the fact that one of the primary uses of the microwave is heating of agarose for the casting of electrophoresis gels. User’s should be aware that previous, possibly less careful, operators may have used the microwave in conjunction with hazardous chemicals (as described later) and as such operation of the microwave using gloves is recommended in any case.
Spectrophotometer
The spectrophotometer is a device that measure the absorbance of a given liquid sample at various wavelengths. A full description of it’s usage is given in the SOPs. User’s should be aware that this system can generate light of significant intensity in both ultraviolet and infrared wavelengths. While most of the optical path and the bulbs required to generate this light is contained within the system, the user may come into contact with this in the sample measurement compartment of the device. As such it should be operated only with the sample measurement comparment fully closed.
PCR Machine
The Polymerase Chain Reaction (PCR) machine, also known as a thermalcycler, is used to amplified specific sections of template DNA either for diagnostic or molecular cloning purposes. The heating block and underside lid of this machine will typically reach temperatures in the region of 95 to 100degC in a typical amplification program and caution should be exercised when placing and removing samples. Information regarding the current state of the machine including current program and temperature is displayed on the LCD on the front panel of this machine. Full information regarding the use and programming of the Techne thermal cycler can be found in the SOPs.
Electrophoresis Power Supply Unit
The Power Supply Unit (PSU) used to supply a current to the electrophoresis tank (described below) is capable of producing dangerously high voltages and currents. It is typically set to the levels required for DNA electrophoresis in 100mL 1 - 2% w/v agarose gels. Caution should be exercised when plugging in and handling electrodes. Use of a residual current device is recommended when using this or any other electrical device in the laboratory that may pose a risk to operators from electric shock.
Electrophoresis tank and Ethidium Bromide area
The electrophoresis tank is located within the electrophoresis/Ethidium Bromide area of the laboratory bench. It is advised any work carried out within this area is manipulated separately from any work done in other areas. Any consumables used when handling Ethidium Bromide such as gloves and tips should be disposed of before handling anything outside of this area to prevent contamination of the wider lab area with the potentially toxic chemicals used in DNA staining. Ideally separate pipettes should also be used for ethidium bromide work vs other work. A full description of how to set up and run agarose gels for DNA electrophoresis is described in the SOPs.
UV illuminator
Gel electrophoresis separates DNA by size within an electric field. Separation of varying sizes of DNA fragment within an agarose gel allows visualisation of the various sizes of DNA fragment contained within a sample when nucleic acid binding stains are used. The typical stain used is Ethidium Bromide which is a fluorophore that is excited within the UV spectrum (with excitation maxima under 300nm) and emission within the visible spectrum. This presents two main risks to the user: i) As a DNA intercalating agent Ethidium Bromide is potentially mutagenic and should never be allowed to come in contact with the user’s bare skin, this applies through the whole DNA electrophoresis procedure from gel preparation, through electrophoresis and then subsequent gel visualisation. This is therefore also carried out in the electrophoresis/ethidium bromide area of the laboratory bench. ii) UV light can be damaging to exposed surfaces of the body and in extreme circumstances or prolonged use lead to carcinomas or eyesight damage. It is therefore advisable that eye protection be used by all people present within the laboratory when UV gel visualisation is taking place and that any possible contact from UV radiation on the user’s body while manipulating and viewing their gel be minimised through adequate PPE and controlled use of the UV light source.
- UV GEL BOX NEEDS FIXING BEFORE AUDIT!!!!*******
HEPA flow cabinet/area
The HEPA filtered laminar flow unit allows us to work in sterile air in order to prevent contamination of our work such as petri dishes and broths with other microorganisms. Proper usage of the laminar flow area is described in the SOPs. It should be noted that the laminar flow functions in such a way as to protect the user’s work rather than the user and so caution still be taken by the user to maintain the aseptic conditions of anything used within the flow area and in disposal of consumables to assist in containment of biomaterial. See SOPs on aseptic technique.
Hot plate/Steam cooker/steriliser/autoclave
The sterilisation unit is used to destroy any potential microorganisms that might contaminate media, reagents and consumables to be used aseptically in micro and molecular biological procedures. High temperature and pressure is used to kill contaminants and the main risk to the user is from the heat of the metal pressurised unit during sterilisation and any vented steam. The system itself is unpowered and is heated using the hot plate which itself becomes hot enough to cause serious burns. Thermal gloves are available for handling the autoclave and material processed by it. It is recommended that the autoclave and contents are allowed to cool for a while before handling. The correct procedures for using the autoclave/steriliser to prepare media and destroy GM waste are described in the SOPs.
Sink area
The sink area should remain clear. All glassware should be kept clean and out of the way.
Chemicals and Storage
As per chemical list, and MSDS by chemical
Incubator
While the incubator itself doesn’t pose much risk due to typically operating in the temperature required for incubating bacteria care should be taken when moving samples to and from the incubator as per handling biomaterial mentioned above.
Centrifuges
The laboratory has a number of centrifuges available for use. The Jouan is a larger devide which can take 50ml tubes and larger containers if fitted with buckets and can spin up to 10,000 rpm. The Henle and MSE microcentaur can spin up to higher rpm with smaller samples. These centrifuges will not operate in their normal state without a closed lid preventing the user from coming into contact with the moving parts. There are two blood centrifuges which spin in the region of 1000 to 2000 rpm while much slower can be operated with open lids however this is strictly advised against for safety reasons. All centrifuges should be cleaned after use for the purpose of biosafety and containment as samples centrifuged at high speeds can leak producing contamination.
Refrigerator, Freezer and Sample Storage
The refrigerator maintains samples and reagents in the area of 4degC such as bacterial plates, and reagents for transformation. Due to the presence of biomaterial in the refrigerator caution should be exercised to prevent contamination of the user. The freezer contains longer term bacterial stocks and enzymatic reagents and similar caution should be taken. Specific details for the handling of the various chemicals and reagents stored in the freezer and refrigerator can be found in the chemicals and storage list above. Specific SOPs cover the storage and recovery of frozen bacterial stocks and procedures requiring the usage of frozen and refrigerated reagents.